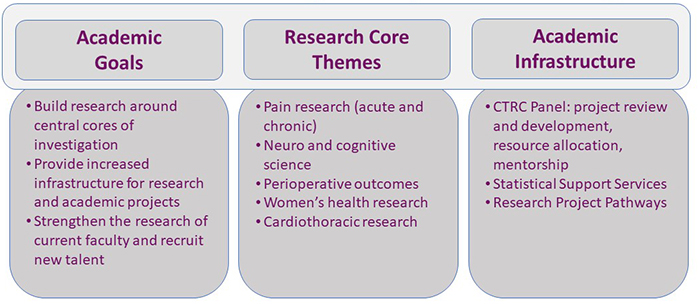
The CTRC’s purpose is to advance the development of successful, innovative, clinical and translational research in support of our department’s academic goals. The CTRC fosters the development of young faculty in our department in research and new grant submissions, oversees the development of research infrastructure and physician-scientist support programs, and reviews research proposals for departmental support.
INVESTIGATOR RESOURCES
We place a high value on research and innovation in each of our clinical divisions. Providing faculty and trainees with the tools and support to achieve success in their research endeavors is a top priority. To aid faculty and trainees, we have many departmental resources to assist with various aspects of research, including statistical support, IT and data analytics, and a pool of dedicated clinical research staff to help with Institutional Review Board submissions, subject enrollment, data collection, and data entry. The pathways below offer specific guidance to faculty and trainees in our department on how to start a research project and illustrates the process for industry-sponsored, grant-funded, and non-funded investigator-initiated clinical trials.
INDUSTRY-SPONSORED TRIALS
This pathway is a phased approach to illustrate the steps required to develop an investigator-initiated protocol/budget with the goal of obtaining industry funding to support the trial. This includes the process for receiving CTRC input and approval, generating a non-disclosure agreement and Clinical Trial Agreement with the University of Pittsburgh’s Office of Sponsored Projects, submission to the Institutional Review Board, ClinicalTrials.gov registration, and obtaining fiscal approval to conduct the trial.
View the industry-sponsored trials pathway
GRANT-SPONSORED TRIALS
This pathway illustrates the steps required to apply for grant funding as well as the process of working with the Office of Sponsored Projects to ensure their timely approval of all required documents.
Coming Soon
NON-FUNDED TRIALS
This pathway provides guidance on how to obtain approval from the CTRC to utilize the departmental resources necessary to implement a study protocol. This pathway also includes the next steps in terms of Institutional Review Board and ClinicalTrials.gov submissions, as well as the logistic steps to implement the trial once approvals are in place.
View the non-funded trial path
CLINICAL DATABASE RESEARCH
This pathway provides the proper steps to submit a request to access our clinical data warehouse to extract research variables for an approved research project. This tool is also instrumental in helping the investigator to understand the types of variables that exist in the database toolkit and the timelines expected with each type of variable extraction.
Database Request (PDF)
TRAINEE GUIDELINES FOR RESEARCH/SCHOLARLY ACTIVITY
These guidelines are designed to assist trainees of all levels (medical students, residents, and fellows) in academic pursuits. Pathways are provided for multiple project types, with tools to help trainees navigate the process of working with a faculty mentor in developing and implementing their research projects.
